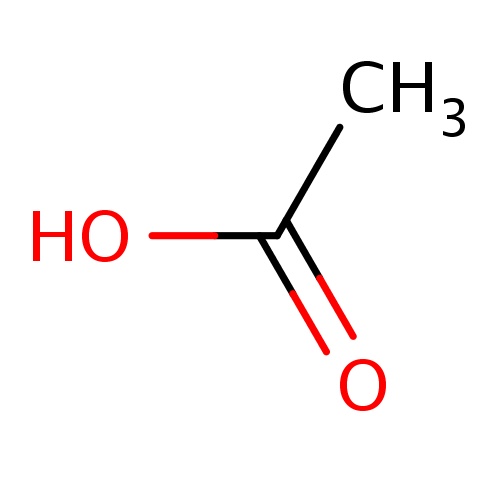
Common name
acetic acid
IUPAC name
acetic acid
SMILES
CC(=O)O
Common name
acetic acid
IUPAC name
acetic acid
SMILES
CC(=O)O
INCHI
InChI=1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4)
FORMULA
C2H4O2

Common name
acetic acid
IUPAC name
acetic acid
Molecular weight
60.052
clogP
-0.484
clogS
0.534
Frequency
0.0687
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
37.3
Number of Rings
0
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01378 | Acetylcysteine |
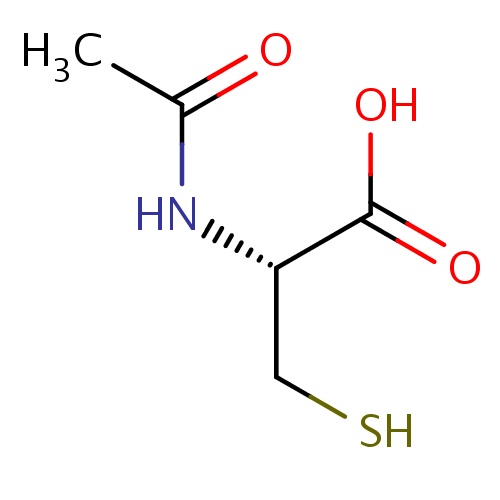
|
Antiviral Agents; Free Radical Scavengers; Antidotes; Expectorants; Mucolytics; Cough and Cold Preparations; Respiratory System; Ophthalmologicals; Sensory Organs; | Acetylcysteine is used mainly as a mucolytic and in the management of paracetamol (acetaminophen) overdose. |
| FDBD01381 | Icatibant |
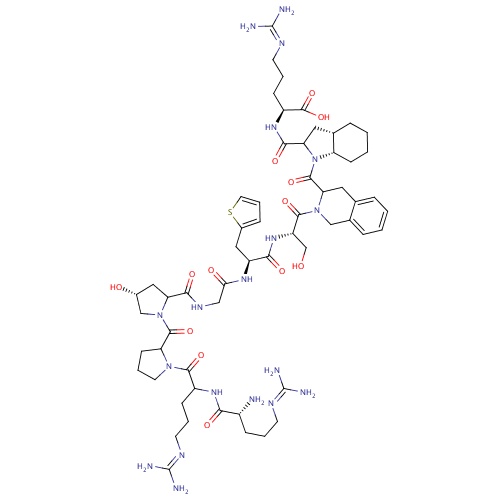
|
Anti-Inflammatory Agents, Non-Steroidal; Adrenergic beta-Antagonists; Blood and Blood Forming Organs; Drugs Used in Hereditary Angioedema; Bradykinin B2 Receptor Antagonists; | Approved for use in acute attacks of hereditary angioedema (HAE). Investigated for use/treatment in angioedema, liver disease, and burns and burn infections. |
| FDBD01402 | Alvimopan |

|
Gastrointestinal Agents; Alimentary Tract and Metabolism; Drugs for Constipation; Peripheral Opioid Receptor Antagonists; | Used to accelerate the time to upper and lower gastrointestinal recovery following partial large or small bowel resection surgery with primary anastomosis. Also investigated for use in the treatment of pain (acute or chronic). |
| FDBD01403 | Levocetirizine |
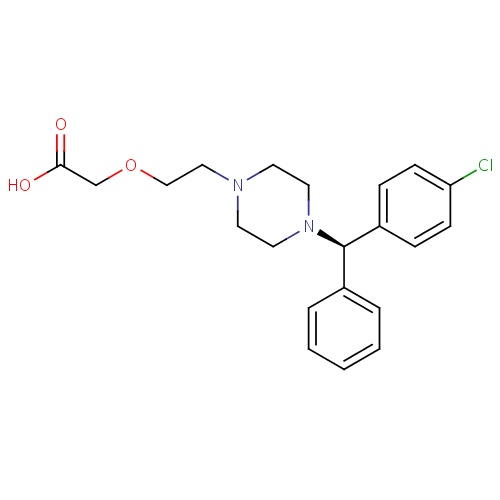
|
Respiratory System; Antihistamines for Systemic Use; Piperazine Derivatives; CYP3A4 Inhibitors; | Levocetirizine is indicated for the relief of symptoms associated with allergic rhinitis (seasonal and perennial) in adults and children 6 years of age and older. |
| FDBD01411 | Ambrisentan |

|
Antihypertensive Agents; Cardiovascular System; Antihypertensives for Pulmonary Arterial Hypertension; Cytochrome P-450 CYP2C19 Inducers; CYP3A4 Inhibitors; | Ambrisentan is indicated for treatment of idiopathic (‘primary |
| FDBD01431 | Ethanolamine Oleate |
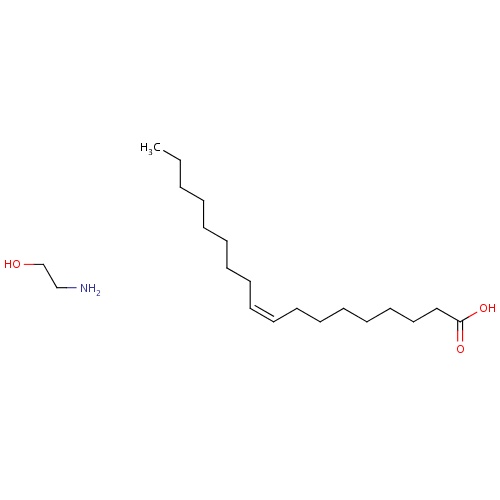
|
Sclerosing Solutions; Cardiovascular System; Vasoprotectives; Sclerosing Agents for Local Injection; Antivaricose Therapy; CYP2E1 Inhibitors; CYP2E1 Inducers; CYP2E1 Inducers (strong); | For the treatment of patients with esophageal varices that have recently bled, to prevent rebleeding. |
| FDBD01435 | Dabigatran etexilate |
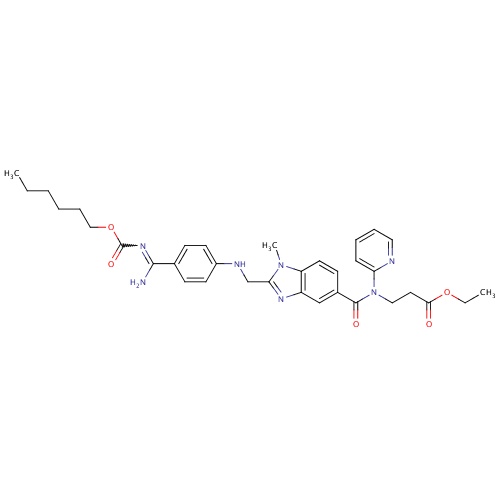
|
Antithrombins; Direct Thrombin Inhibitors; Antithrombotic Agents; Blood and Blood Forming Organs; | Dabigatran is indicated for the prevention of venous thromboembolic events in patients who have undergone elective hip or knee replacement surgery (based on RE-NOVATE, RE-MODEL, and RE-MOBILIZE trials). In 2010, it was approved in the US and Canada for prevention of stroke and systemic embolism in patients with atrial fibrillation (approval based on the RE-LY trial). Contraindications: severe renal impairment (CrCL . |
| FDBD01443 | Gadobutrol |
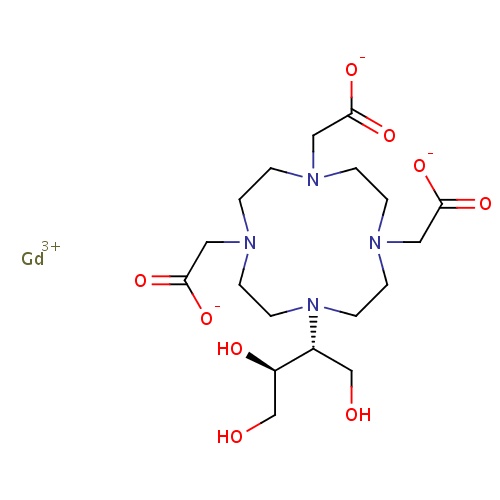
|
Contrast Media; Paramagnetic Contrast Media; Magnetic Resonance Imaging Contrast Media; | For diagnostic use only. Indicated for adults and children age 2 and over for contrast enhancement during cranial and spinal MRI, and for contrast-enhanced magnetic resonance angiography (CE-MRA). Gadobutrol is particularly suited for the detection of very small lesions and for the visualization of tumors that do not readily take up contrast media. It may be a desired agent when the exclusion or demonstration of an additional pathology may influence the choice of therapy or patient management. It may also be suitable for perfusion studies in the diagnosis of stroke, detection of focal cerebral ischemia, and in studies of tumor perfusion. |
| FDBD01445 | Gadofosveset trisodium |
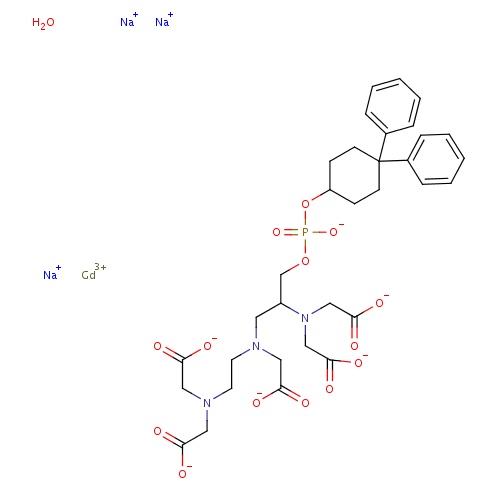
|
Contrast Media; Diagnostic Agents; Paramagnetic Contrast Media; Magnetic Resonance Imaging Contrast Media; | Gadofosveset trisodium is indicated for use as a contrast agent in magnetic resonance angiography (MRA) to evaluate aortoiliac occlusive disease (AIOD) in adults with known or suspected peripheral vascular disease. |
| FDBD01466 | Glycine betaine |
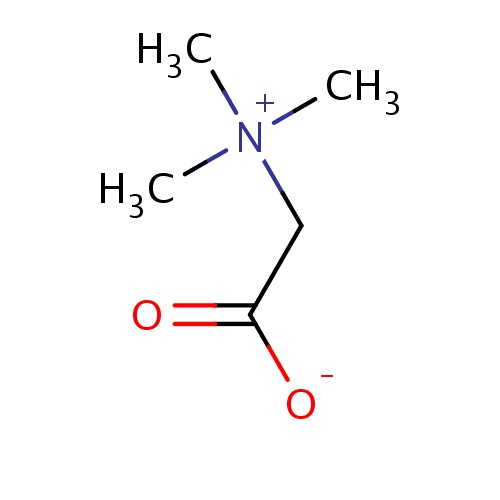
|
Gastrointestinal Agents; Lipotropic Agents; Alimentary Tract and Metabolism; Amino Acids and Derivatives; Acid Preparations; Digestives, Incl. Enzymes; | Betaine is indicated for the treatment of homocystinuria to decrease elevated homocysteine blood levels. Included within the category of homocystinuria are deficiencies or defects in: 1. cystathionine beta-synthase (CBS), 2. 5,10-methylenetetrahydrofolate reductase (MTHFR), 3. cobalamin cofactor metabolism (cbl). |
200 ,
21
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2woq_ligand_frag_4.mol2 | 2woq | 1 | -6.05 | CC(=O)O | 4 |
| 1r9l_ligand_frag_1.mol2 | 1r9l | 1 | -6.04 | CC(=O)O | 4 |
| 4euo_ligand_frag_2.mol2 | 4euo | 1 | -6.04 | CC(=O)O | 4 |
| 2nt7_ligand_frag_3.mol2 | 2nt7 | 1 | -6.02 | CC(=O)O | 4 |
| 2b4l_ligand_frag_1.mol2 | 2b4l | 1 | -6.00 | CC(=O)O | 4 |
| 1d6s_ligand_frag_1.mol2 | 1d6s | 1 | -5.99 | CC(=O)O | 4 |
| 1ibc_ligand_frag_15.mol2 | 1ibc | 1 | -5.99 | CC(=O)O | 4 |
| 1nms_ligand_frag_2.mol2 | 1nms | 1 | -5.99 | CC(=O)O | 4 |
| 2vl1_ligand_frag_2.mol2 | 2vl1 | 1 | -5.99 | CC(=O)O | 4 |
1717 ,
172

