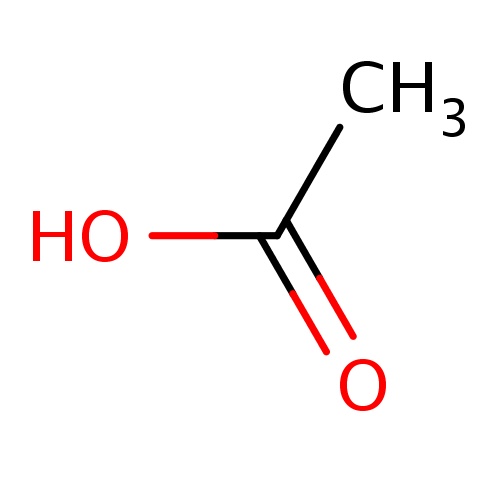
Common name
acetic acid
IUPAC name
acetic acid
SMILES
CC(=O)O
Common name
acetic acid
IUPAC name
acetic acid
SMILES
CC(=O)O
INCHI
InChI=1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4)
FORMULA
C2H4O2

Common name
acetic acid
IUPAC name
acetic acid
Molecular weight
60.052
clogP
-0.484
clogS
0.534
Frequency
0.0687
HBond Acceptor
2
HBond Donor
1
Total PolarSurface Area
37.3
Number of Rings
0
Rotatable Bond
0
| Drug ID | Common name | Structure CAS | Compound class | Therapeutic area |
|---|---|---|---|---|
| FDBD01471 | Bendamustine |
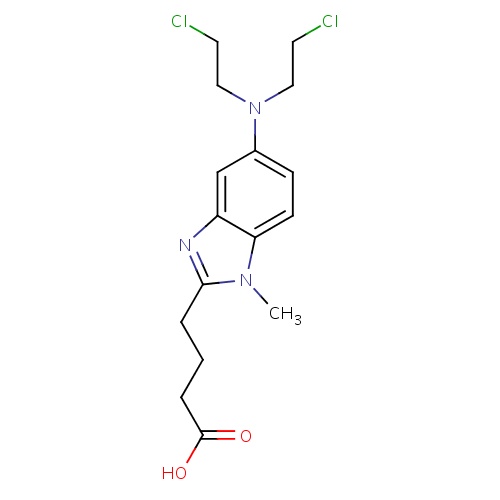
|
Antineoplastic Agents; Antineoplastic Agents, Alkylating; Alkylating Agents; Antineoplastic and Immunomodulating Agents; Nitrogen Mustard Analogues; | Bendamustine is indicated for treatment of chronic lymphocytic leukemia (CLL). |
| FDBD01474 | Cabazitaxel |
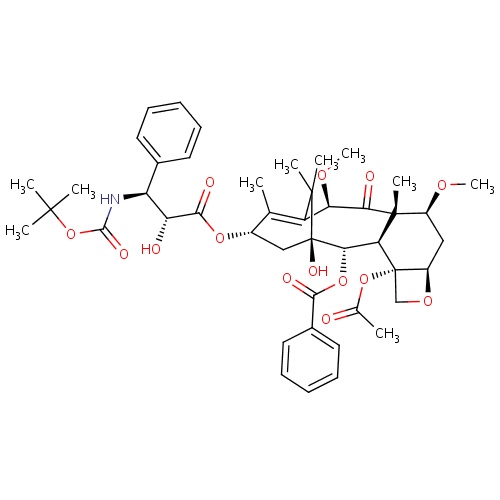
|
Antineoplastic Agents; Immunosuppressive Agents; Antineoplastic and Immunomodulating Agents; Taxanes; Cytochrome P-450 CYP2C8 Inhibitors; Cytochrome P-450 CYP2C8 Inducers; CYP3A4 Inhibitors; | For treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing treatment regimen. |
| FDBD01476 | Carglumic Acid |
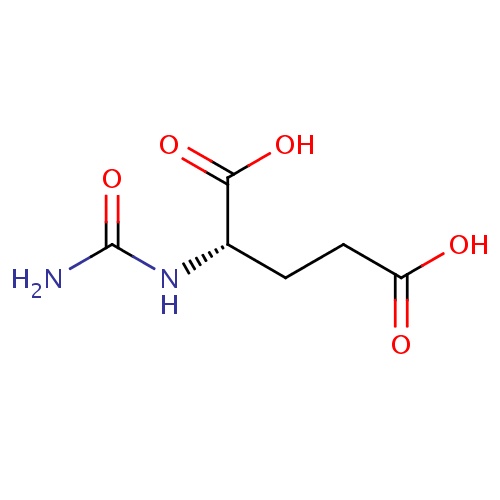
|
Alimentary Tract and Metabolism; Amino Acids and Derivatives; | For the treatment of acute and chronic hyperammonaemia in patients with N-acetylglutamate synthase (NAGS) deficiency. This enzyme is an important component of the urea cycle to prevent build up of neurotoxic ammonium in the blood. |
| FDBD01477 | Chenodeoxycholic acid |
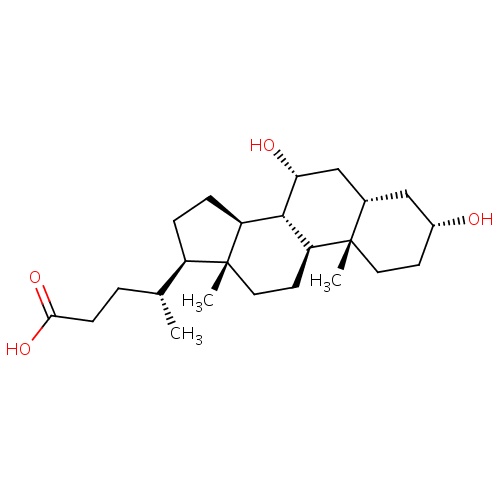
|
Gastrointestinal Agents; Cathartics; Alimentary Tract and Metabolism; Bile and Liver Therapy; Bile Acid Preparations; Bile Therapy; CYP3A4 Inhibitors; | Chenodiol is indicated for patients with radiolucent stones in well-opacifying gallbladders, in whom selective surgery would be undertaken except for the presence of increased surgical risk due to systemic disease or age. Chenodiol will not dissolve calcified (radiopaque) or radiolucent bile pigment stones. |
| FDBD01484 | Histrelin |
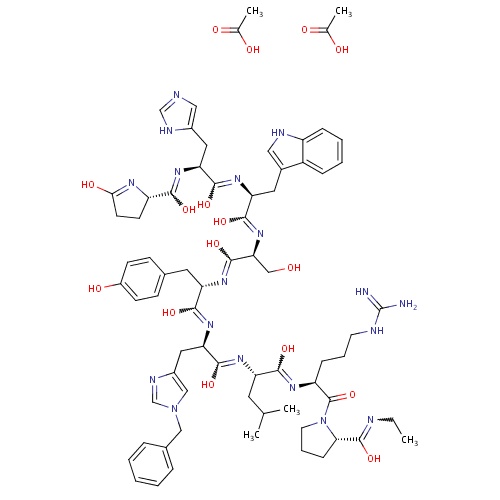
|
Histrelin acetate has two indications | |
| FDBD01500 | Pralatrexate |
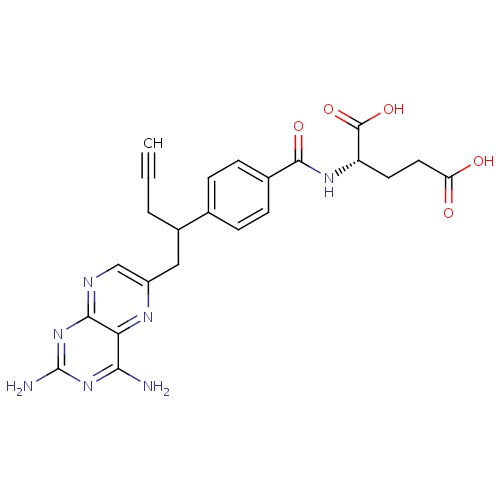
|
Antineoplastic Agents; Immunosuppressive Agents; Antimetabolites; Antineoplastic and Immunomodulating Agents; Folic Acid Analogues; | Treatment of relapsed or refractory peripheral T-cell lymphoma. |
| FDBD01503 | Sodium phenylbutyrate |
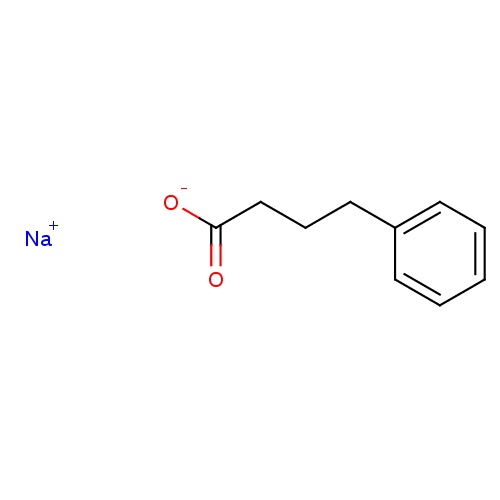
|
Alimentary Tract and Metabolism; Various Alimentary Tract and Metabolism Products; | |
| FDBD01505 | Tiopronin |
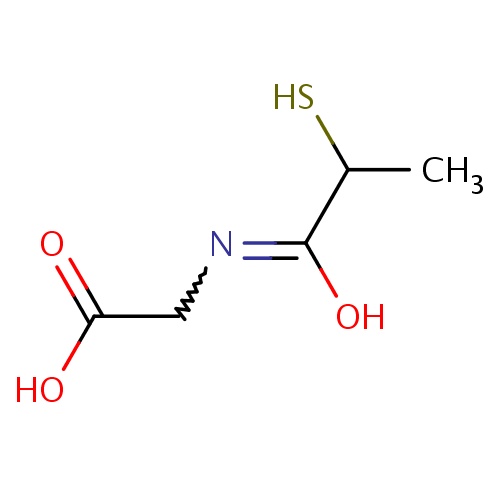
|
Mucolytics; Cough and Cold Preparations; Respiratory System; Genito Urinary System and Sex Hormones; Urinary Concrement Solvents; Urological Agents; | Tiopronin is indicated for the prevention of kidney stone formation in patients with severe homozygous cystinuria consisting of a urinary cystine concentration greater than 500 mg/day, and who have failed treatment with non-pharmacological measures of increased fluid intake, decreased sodium and protein intake, and urine alkalinization. |
| FDBD01540 | Spaglumic Acid |
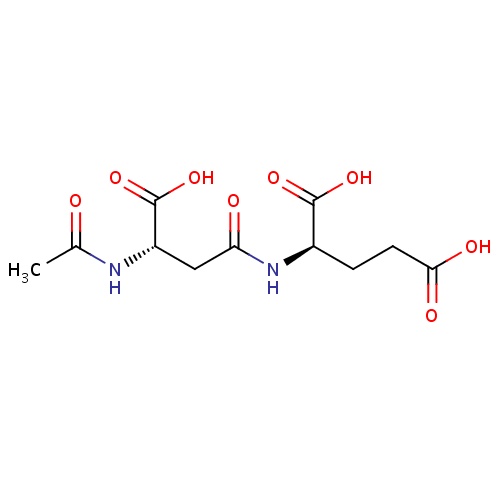
|
Mast Cell Stabilizers; Respiratory System; Ophthalmologicals; Sensory Organs; Nasal Preparations; Decongestants and Antiallergics; Antiallergic Agents, Excl. Corticosteroids; | Used in patients with allergic conjunctivitis. |
| FDBD01541 | Acetylcarnitine |
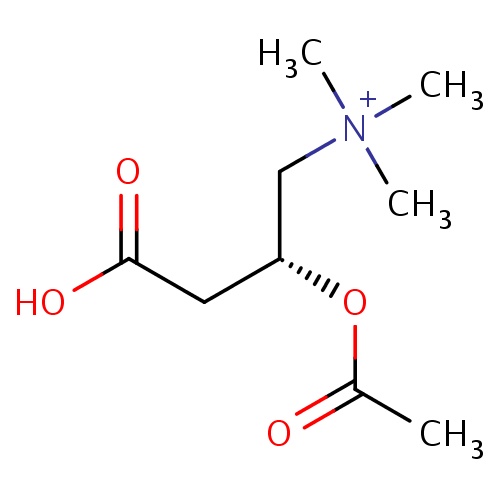
|
Acetylcarnitine is not approved for any indication in the United states and Canada, but it is approved and indicated in Italy for cerebrovascular disorders, mental function disorders, peripheral nerve disorders, diabetic neuropathy, and nutritional supplementation; Portugal for mental function disorders; Argentina for cerebral vasculopathy, nutritional supplementation, and peripheral neuropathy; Chile for dementia; Philippines for cerebrovascular disorders and mental function disorders; Australia for nutritional supplementation; and India for nutritional supplementation to increase sperm count. Acetylcarnitine also has several potential therapeutic indications for which it is still being investigated: in Norway, acetylcarnitine is in a phase IV trial for prophylactic treatment of migraines; in Italy acetylcarnitine is in a phase II trial for use in patients with type 2 Diabetes Mellitus, a phase III trial for alleviating fatigue in patients with chronic hepatitis C, and for use in patients with Minimal Hepatic Encephalopathy; in the United States acetylcarnitine is in a phase II trial for the neurodegenerative disorder Progressive Supranuclear Palsy, a phase II and III trial for reducing peripheral neuropathy in cancer patients as an adjunct to chemotherapy, a phase I and II trial for treating patients in septic shock, a phase II trial for bipolar depression, a phase II trial to reduce oxidative stress in patients with Sickle Cell disease, a phase I and II trial for chronic fatigue syndrome, and a study for preventing nerve damage in HIV patients; in China acetylcarnitine is in a phase III trial for reducing peripheral neuropathy in cancer patients as an adjunct to chemotherapy; in the United Kingdom acetylcarnitine is being investigated for preventing nerve damage in HIV patients; and in Israel acetylcarnitine is being studied for the treatment of male infertility. |
200 ,
21
| FRAGNAME | PDBID | SIMILIRITY | XSCORE | SMILE | HAC |
|---|---|---|---|---|---|
| 2woq_ligand_frag_4.mol2 | 2woq | 1 | -6.05 | CC(=O)O | 4 |
| 1r9l_ligand_frag_1.mol2 | 1r9l | 1 | -6.04 | CC(=O)O | 4 |
| 4euo_ligand_frag_2.mol2 | 4euo | 1 | -6.04 | CC(=O)O | 4 |
| 2nt7_ligand_frag_3.mol2 | 2nt7 | 1 | -6.02 | CC(=O)O | 4 |
| 2b4l_ligand_frag_1.mol2 | 2b4l | 1 | -6.00 | CC(=O)O | 4 |
| 1d6s_ligand_frag_1.mol2 | 1d6s | 1 | -5.99 | CC(=O)O | 4 |
| 1ibc_ligand_frag_15.mol2 | 1ibc | 1 | -5.99 | CC(=O)O | 4 |
| 1nms_ligand_frag_2.mol2 | 1nms | 1 | -5.99 | CC(=O)O | 4 |
| 2vl1_ligand_frag_2.mol2 | 2vl1 | 1 | -5.99 | CC(=O)O | 4 |
1717 ,
172

